
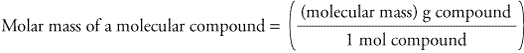
To get the formula from the triangle, cover up the value you want to calculate and use the remaining two values. To remember the formulas in this section, you can use the formula triangles. For example, about 98.9% of naturally occurring carbon is 12C ('Carbon 12') and about 1.1% is 13C so carbon has an atomic weight of \begin Formula Triangles The atomic weight (or atomic mass) of an element tells us on average how much one atom of a given element weighs, taking into account typical proportions of isotopes. Mass of 3.9 × 10 25 molecules of H 2 O = 1167 g.Īnswer: the mass of 3.9 × 10 25 molecules of H 2 O is 1167 g.Contents Toggle Main Menu 1 Atomic Weight 2 Molecular Weight 3 Moles 4 Solutions 4.1 1) Molarity 4.2 2) Mass Concentration 4.3 A note on units for concentrations 4.4 Diluting Solutions 5 Test yourself Atomic Weight Dispersion forces are decisive when the difference is molar mass or. The question is asking about the mass of 3.9 × 10 25 molecules of water. For example, water has London dispersion, dipole-dipole, and hydrogen bonds. So the molar mass of H 2 O = 2(Molar mass of hydrogen atoms) + 1(Molar mass of oxygen atoms). We know that there are two hydrogen atoms and one oxygen atom for every H 2 0 molecule.
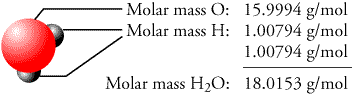
To find the mass of 3.9 × 10 25 H 2 O molecules, first we have to find the mass of one molecule of H 2 O. Q.3 Calculate the mass in grams of 3.9 × 1025 H2O molecules Step 1 Mass of 9.25 10 22 molecules of H 2 O = 2.768 g.Īnswer: the mass of 9.25 10 22 molecules of water is 2.768 g. The question is asking about the mass of 9.25 10 22 molecules of water. Mass of one mole of H 2 0 (Water) = 18.02 g So, the molar mass of H 2 O = 2(Molar mass of hydrogen atoms) + 1(Molar mass of oxygen atoms) To find the mass of 9.25 10 22 molecules of water, firstl we have to find the mass of one molecule of water (H 2 O). Q.2 What is the mass of 9.25 1022 molecules of water? Step 1 The question is asking about the mass of 2.5 × 10 9 molecules of water. Molar mass of H 2 0 =18.02 g/mol Mass of one mole of H 2 0 (Water) = 18.02 g So, the molar mass of H 2 O = 2(Molar mass of hydrogen atoms) + 1(Molar mass of oxygen atoms). To find the mass of 2.5 × 10 9 H 2 O molecules, first we have to find the mass of one molecule of H 2 O. More examples Q.1 Calculate the mass in grams of 2.5 × 109 H2O molecules Step 1 Mass of 1.00×10 24 molecules of H 2 O = 29.9 g.Īnswer: the mass of 1.00×10 24 molecules of water is 29.9 g. 1 H2O is equal to 18.02 g/mol Molar mass units Gram per mole (g/mol) 18.02 Kilogram per mole (kg/mol) 0.02 Standard atomic weight Hydrogen (H) 17.87 Oxygen (O) 1.13 Sulfur (S) 0. Mass of 1.00×10 24 molecules of H 2 O = 2.99 x 10 1 g. Water molecule (H2O - Molecular mass), molar mass Type the number of Water molecule (H2O) you want to convert in the text box, to see the results in the table. The question is asking about the mass of 1.00×10 24 (a septillion) molecules of water. Mass of X molecules of H 2 O = (Mass of a mole of H 2 O molecules) (X molecules)/ 6.022 x 10 23 Mass of X molecules of H 2 O / X molecules = Mass of a mole of H 2 O molecules / 6.022 x 10 23 This relation is then used to ‘convert’ a number of H 2 O molecules to grams by the ratio: One mole of H 2 O is 6.022 x 10 23 molecules of H 2 O (Avogadro’s number). Molar mass of H 2 0 =18.02 g/mol Mass of one mole of H 2 0 (Water) = 18.02 g Step 2 So, the molar mass of H 2 O = 2 (Molar mass of hydrogen atoms) + 1(Molar mass of oxygen atoms).


 0 kommentar(er)
0 kommentar(er)
